Case Studies
At Sensors Summit 2019, the presentation, The Process to Commercialize a Medical Device for Scale, Quality, Performance and Profit, was given as a tutorial. A white paper was also written. It is often mistaken that the commercialization process begins with a design, a lab prototype and experimental testing. So often entrepreneurs and companies come with design, prototype and validation in hand without a customer or an understanding of their needs, consideration for manufacturing and quality nor any evaluation of a supply base. This presentation challenges this thinking and presents an alternative process that starts with an idea accompanied by the end customer’s specifications, a thorough review of the method for manufacturing, definition of a quality plan and an understanding of your validation requirements at the component and device level. There should also be a consideration of your potential supply base and their capabilities. Furthermore, a plan should be developed for the path through the FDA approval process.

At Sensors Expo 2018, the presentation, Rapid Prototyping of Wireless Sensor Solutions for Future Applications, was given at the pre-conference symposium, MEMS and Sensor Technologies Coming to an LoT Solution Near You. Many new sensor applications inherently require wireless technology or use it to simplify implementation and lower cost in the field. Rapid, high performance and reasonably priced prototypes of these ideas facilitate the collection of data that demonstrate proof of concept necessary to solicit investor funding or obtain management approval to proceed. This presentation covers solutions to hardware challenges that require integration of multiple hardware components. Furthermore, it highlights equally important software solutions using a combination of off-the-shelf modules and custom code to get desired function in weeks not months or years. The presentation concludes with a case study of a wireless medical sensor developed with a world renown surgeon that demonstrates this process and reviews a future application of this technology.

At BioMEMS 2013, the presentation, The Revolutionary Change in Sports from MEMS and Sensor Enabled Products, was given to highlight how MEMS and sensors in sports are seeing explosive growth. Enabling factors such as their unobtrusive micro size, reduced cost, accuracy, use of smart algorithms, low power consumption, wireless connectivity, simplified interfaces for data interpretation and mobile computing are making this available to everyone not just elite professional athletes in an expensive laboratory setting. As a result, revolutionary changes are occurring in how we learn, our overall understanding of humans in sports and our resulting behavior enabling us to increase performance and achieve our goals in shorter intervals. In addition, sensors and MEMS products are providing useful data to help engineers make sport protection gear more effective, people lose unwanted pounds and athletes avoid health danger zones.

This presentation, Medical Sensors – Enhancing Human Performance and a Peak into the Future, was given at the Human Performance Workshop at Kent State University. Sensors in the medical field are seeing explosive growth. This talk reviews four case studies of innovative products playing a significant role in enhancing human performance. Topics include successful weight loss, digital medicine, sight restoration and over coming paralysis. For each topic, an overview of the product and sensor technology will be explored. In addition, we’ll take a journey into the future to see how medical, engineering and scientific collaboration can further enhance human performance. Some of the outstanding work discussed was performed with off-the-shelf or suboptimal equipment. imagine what can happen when collaboration occurs to provide optimal solutions.
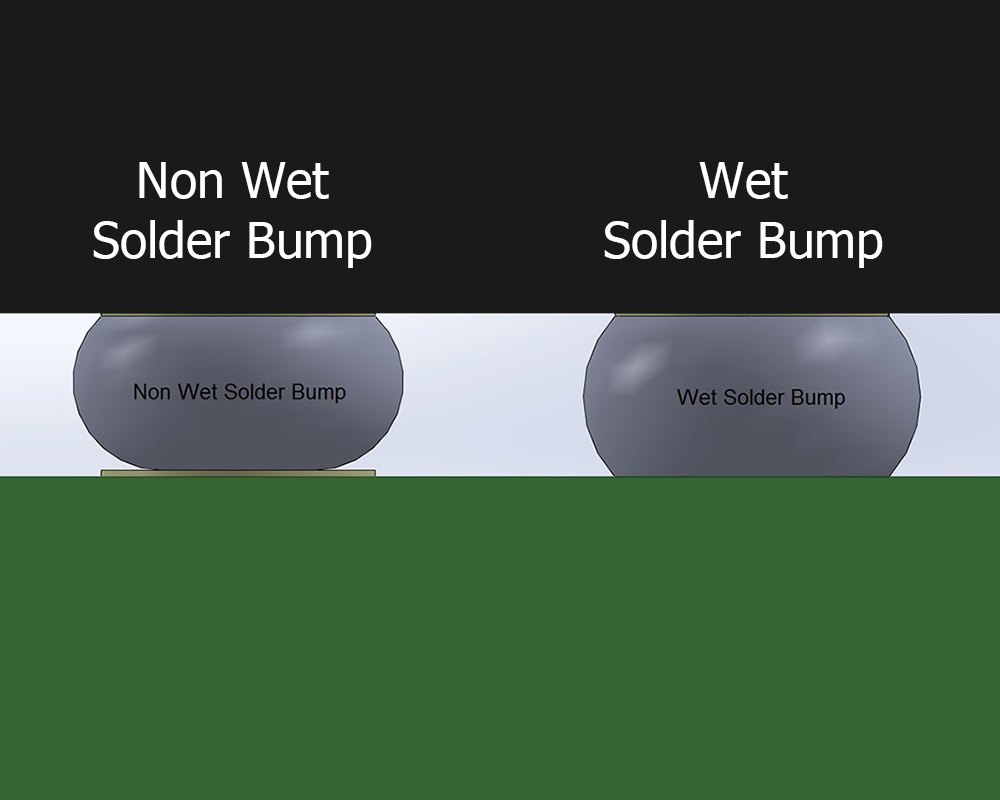
In the January-February 2013 edition of Chip Scale Review Magazine, David contributed a Guest Editorial, Robust Process Solutions for MEMS Flip Chip Applications With Medium Density Lead Free Solder Interconnects. The article reviews the challenges in electrical interconnection for increasingly smaller MEMS flip chip applications using lead free solder for medium density I/O, size sensitive medical applications. With the proliferation of MEMS in sensor products in a variety of applications, packaging challenges continue to be at the forefront of successful production launches. Furthermore, package shrinkage, cost pressure and rapid time to market magnify these challenges because traditional approaches can no longer meet these constraints.
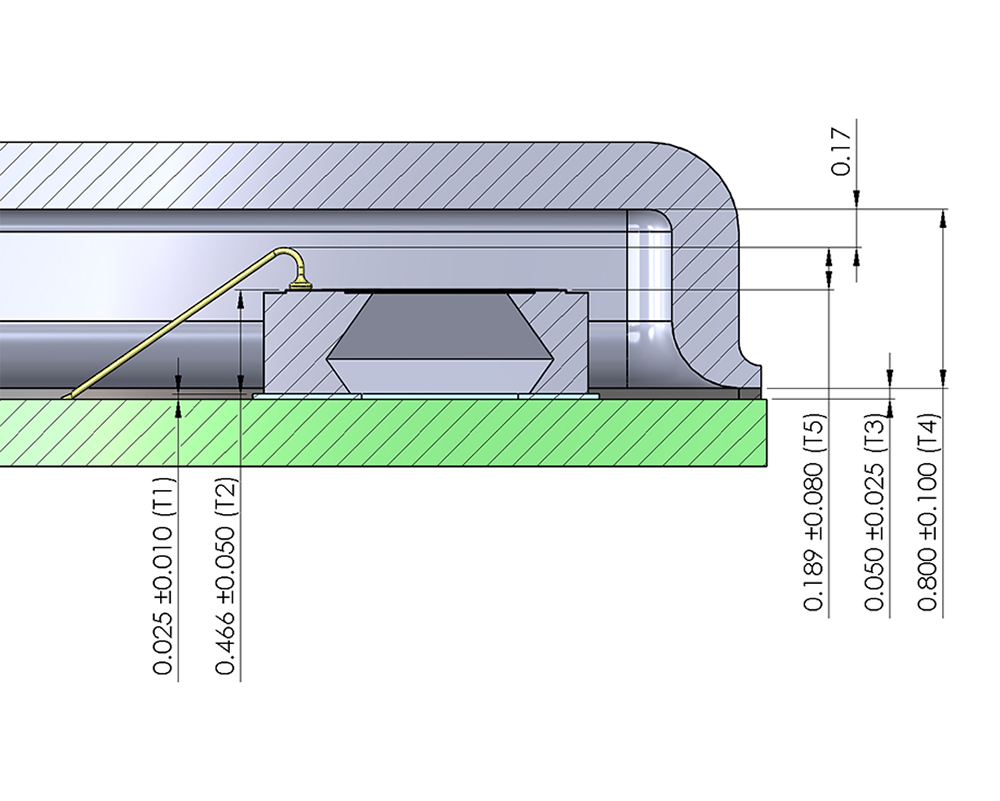
Check out David’s blog on MEMS new product development in Solid State Technology. This monthly series discussed critical aspects of developing new MEMS products for commercialization.
- A Sellable Plan
- Importance of First Prototype
- Critical Design and Process Steps for Successful Prototypes (Part 1)
- Critical Design and Process Steps for Successful Prototypes (Part 2)
- The Technology Development Process and Design Review Checklist
- Necessary Attributes for a MEMS Engineer for New Product Development
- Importance of Product Validation
David published an article, BioMEMS: Navigating the Medical Device FDA Approval Process, in MEMS Journal reaching 31,000+ subscribers. With major advances in MEMS and nanotechnology and their proliferation into medicine, it is important to have a working knowledge of the critical steps in obtaining U.S. Food and Drug Administration (FDA) approval for commercialization. An understanding of the core elements and expedited review opportunities for medical devices provides entrepreneurs a key advantage in decision making, development … Read More.
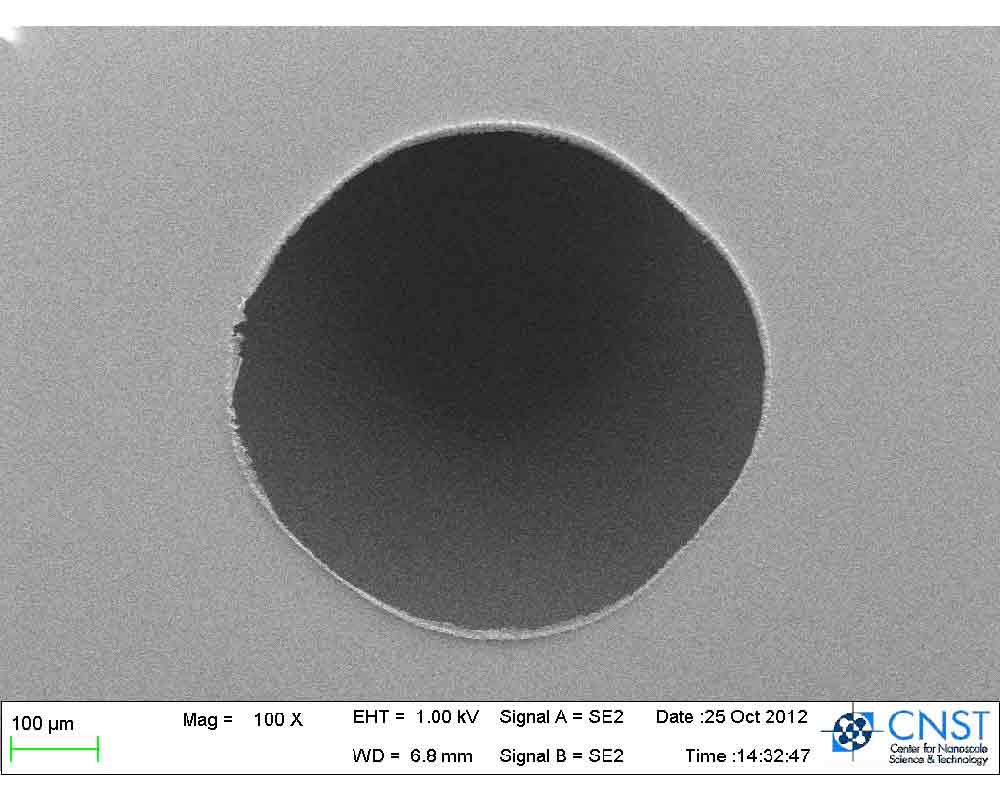
DiPaola Consulting was featured in the Fall Issue of CNST News by the NIST Center for Nanoscale Science and Technology in an article, Entrepreneur Builds MEMS Pressure Sensor to Improve Automotive Transmissions. The pressure sensor was designed to work inside of an automatic transmission to provide closed loop control. The benefit of closed loop control is less complex and more efficient calibration with reduced manpower and improved shift feel. The advantage of using NIST’s CNST is that a prototype can be fabricated at a fraction of the cost of a pure play foundry to demonstrate proof of concept. The sensor was built on a 150 mm silicon-on-insulator (SOI) wafer using a deep reactive ion etched cavity to create the pressure port. The first iteration of the design was to validate the mechanical features. Next all of the electrical features such as the full wheat stone bridge will be incorporated. The full cost of the project was anticipated to be $30,000.

DiPaola Consulting is a leading expert in MEMS integration for sensor products. A webinar, Concurrent Design to Achieve High Performance and Low Cost: Case Study of a MEMS Automotive Pressure Sensor Design, highlights MEMS integration in practice. Presented by David, this webinar was conducted for the MEMS Industry Group (MIG) as part of the educational series leading to the M2M Forum in May 2012. To access the webinar, you need to be a MIG member and it can be found under Resource Library (search title). For non MIG members, you can access the presentation here.
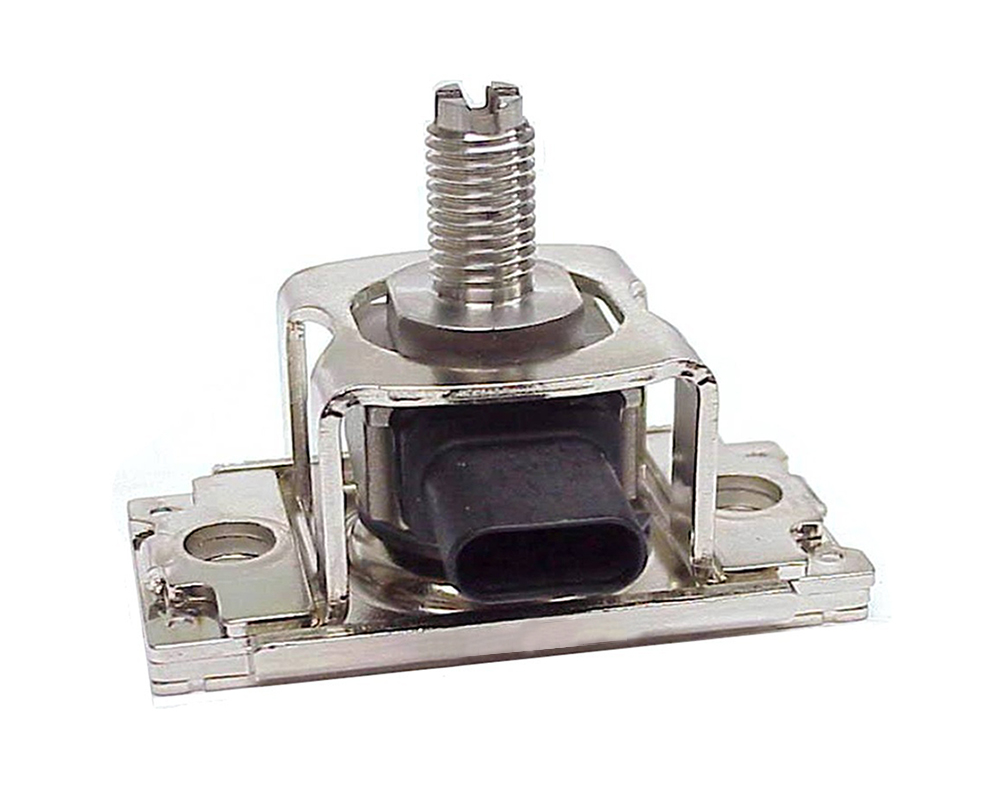
- Patent No. 6,672,170, Hermetic Pressure Transducer, issued 2004
- Patent No. 6,763,724, Hermetic Pressure Transducer, issued 2004
- Patent No. 7,112,749, Sensor Mounting Apparatus for Minimizing Parasitic Stress, issued 2006 (80% contribution to claims)
- Patent No. 7,779,701, Pressure Sensor Apparatus, issued 2010
- Patent No. 7,939,772, Electrical Switch System for Use with Vehicular Transmissions, issued 2011
- Patent No. 8,707,774, Sensor System for Differential Pressure Measurement, issued 2014

David DiPaola and John Brennan presented Selection of Silicone Sealants for Heavy Truck and Off Road Applications at the Society of Plastics engineers. The work summaries testing to screen potential silicone sealants for sensor use in heavy truck and off-road vehicles. The silicone sealants needed work across a wide temperature range (-40 to 160 degrees Celsius) and on multiple surfaces including, plated steal, brass, aluminum and varying grades of plastics. Furthermore the silicone needed to withstand the CTE mismatch between plastic and metal. Both acetic acid and neutral cure room temperature vulcanizing (RTV) silicones were tested. A lap shear test in combination with product testing was conducted. The results showed the neutral cure silicone significantly out performed the acetic acid cure silicone. For full details, please review the presentation.
In new product development, there are several phases including concept, prototype, pilot and production. At the end of each phase, there is a peer and management review of the completed work to determine if critical requirements have been satisfied before proceeding to the next phase. The Design Review Gating Requirements Template, outlines the necessary details from the big picture that need to come together successfully to launch a robust product, on schedule and within budget. Note, this template only presents the design portion of the process.
